Ryder M, Pulcini E, Parker A, James G. Presented at the World Congress on Vascular Access, June 2016.
Summary
The risk of bloodstream infections associated with vascular access devices and needleless valves is a growing concern. Many questions remain regarding the potential infection risks among different types of connectors. Disinfection before access is crucial in preventing the entry of microorganisms, but adherence to this practice is often insufficient. This study, along with its accompanying statistical analysis, compares the rate of bacterial transfer through 20 needleless valves and examines biofilm formation inside the valve, within the catheter connector, and inside the catheter.
The in vitro model was designed to simulate four daily antibiotic infusions using the SASH (saline, antibiotic, saline, and heparin) method. During each five-day study, the connector of each valve was contaminated twice daily with approximately 10^6 colony-forming units (CFU) of Staphylococcus aureus ATCC #6538. After each inoculation, the valves were flushed six times, and cultures were taken from the first and last flushes to count the bacteria. The average logarithmic densities (LD) of CFU inside the valve, within the catheter connector, and inside the catheter were also measured.
Results
Neutron® and MicroClave® valves exhibit the lowest average logarithmic density of bacteria in the flush fluid compared to all other valves (= 3.14 and 3.20 log(CFU/flush)). MicroClave and Neutron did not differ significantly from each other statistically and are the only devices in the group that had a statistically significant, lowest average logarithmic density of bacteria in the flush fluid.
Conclusions
Ryzyko przeniesienia bakterii przez zawór, w łącznik cewnika i światło cewnika do krwioobiegu z zanieczyszczonej powierzchni zaworu zależy od rodzaju zastosowanego zaworu. Analizy regresji sugerują, że log (CFU/złącze) jest najlepszym pojedynczym predyktorem średniej dobowej LD bakterii w popłuczynach (spośród trzech predyktorów: wewnątrz zaworu, w łączniku cewnika i wewnątrz cewnika.
Introduction
Previous studies indicate that the design elements of needleless valves affect the potential for bacteria to transfer from the valve surface to the flow path, catheter connector, and catheter lumen. Biofilm within the lumen is the predominant source of catheter-related bloodstream infections (CRBSI).
Objective
The objective of the study was to compare the rate of bacterial transfer for 20 needleless valves and to compare the formation of biofilm inside the valve, within the catheter connector, and inside the catheter.
Methods
A total of 20 needleless valve designs were evaluated. Four valves of each type were assessed in three independent studies (n=9), with MicroClave serving as the control in each of the 33 series. The valve membrane was contaminated twice daily with approximately 10^6 CFU of Staphylococcus aureus ATCC #6538. The inoculated valve was left to dry for 30 minutes, then connected to a 50 cm polyurethane peripherally inserted central catheter (PICC).
Each catheter-valve set was flushed with 3.0 ml of sterile saline solution, which was collected and cultured on a plate (first flush). The catheter-valve sets were then flushed with sterile saline solution (NS) two more times and blocked with sterile Brain Heart Infusion Broth (BHI) for one hour. Subsequently, they were flushed with NS three more times. The final flush was also cultured on a plate (last flush).
The catheter-valve sets were contaminated for a second time each day after the sixth sterile flushing with saline, followed by a second series of flushing, transfer to a plate, and blocking for a total of 18 valve activations per day, which is considered a routine number of activations in the Intensive Care Unit.
The entire procedure was repeated daily for five days. Except for days 4 and 5, when two catheter-valve sets were sampled for microbiological and destructive microscopic examination.
Statistical analysis was conducted using ANOVA and Tukey tests to determine significant mean differences in the logarithmic density of bacteria in the flush, within the valve, in the catheter connector, and within the catheter among different needleless connectors. Multiple linear regression was employed to determine if any combination of bacterial logarithmic density within the valve, catheter connector, and within the catheter could significantly predict the logarithmic density of bacteria in the flush.
Results
The Neutron and MicroClave valves generally exhibit the lowest mean logarithmic density of bacteria in the flush among all components of the system (=3.14 and 3.20 logarithm (CFU/flush)). MicroClave and Neutron did not significantly differ from each other and are the only devices that are in the group with the smallest significant mean logarithmic density of bacteria after flushing.
In the Q‑Syte® and UltraSite® valves, significantly higher mean LD of bacteria was observed after flushing compared to other types of valves (= 5.37 and 5.15 log (CFU/flush)). Q‑Syte and UltraSite did not differ significantly (p = 0.9101).
TABLE 1: AVERAGE DAILY BACTERIAL DENSITY IN FLUSH SOLUTION.
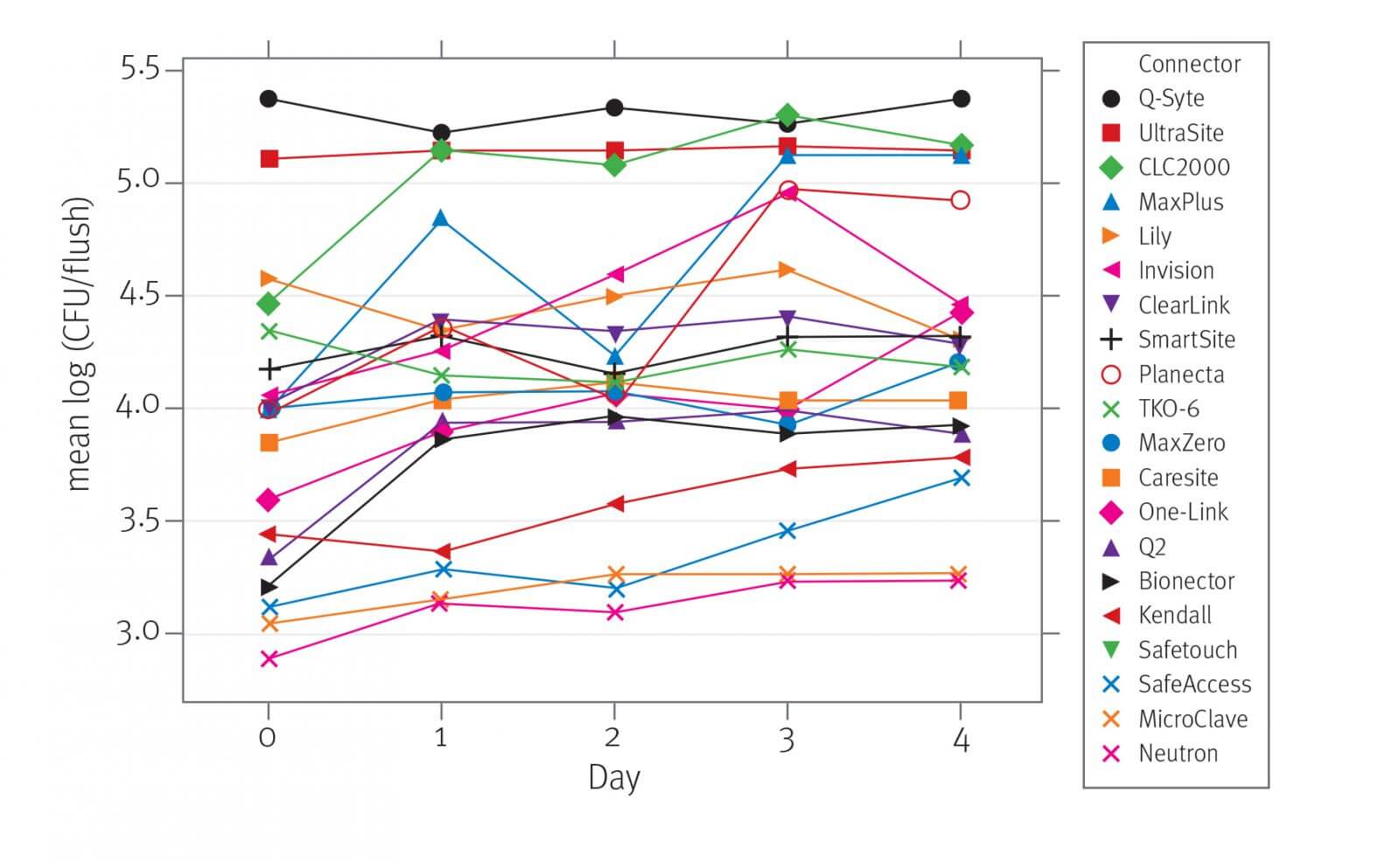
TABLE 2: AVERAGE SQUARE OF FLUSHES ACROSS ALL DAYS AND ALL FLUSHES.
.jpg)
Discussion
The risk of bacterial transfer through the interior of the valve, catheter connector, and inside the catheter into the bloodstream from a contaminated valve surface depends on its type. The study’s findings confirm that the formation of biofilm in the catheter connector and within the catheter lumen may result from bacterial transfer through the needleless valve. Moreover, it has been demonstrated that detached or planktonic bacteria detaching from the biofilm are subsequently introduced into the bloodstream through infusion. Regression analysis indicates that biofilm formation in the valve was the best predictor of the number of bacteria introduced into the bloodstream (R2 = 95%). Thus, the use of a valve with a low rate of microorganism transfer can minimize the risk of bloodstream infection. It also underscores the importance of consistent and effective valve and catheter hub disinfection methods before obtaining access as a key strategy for preventing CRBSI. The data also suggest that the common classification regarding valve characteristics, such as split septum and mechanical valve, is an unreliable approach to device selection based on infection risk.
Conclusions
The risk of bacterial transfer from the contaminated valve surface through the catheter connector and catheter lumen into the bloodstream depends on the type of valve used, and MicroClave and Neutron valves demonstrated significantly lower bacterial transfer rates than any other valves tested. Additionally, the frequency of valve replacement may depend on the potential for bacterial transfer in each device design. Data from this study also suggest that the common classification of split-septum and mechanical valves is too simplistic and unreliable as an approach to device selection based on infection risk.
Bibliography:
- Brown JD, Moss HA, Elliott TSJ. The potential for catheter microbial contamination from a needleless connector. J Hosp Infect. 1997; 36:181–189
- Yebenes J, Delgado M, Sauca G, Serra-Prat M, Solsona M, Almirall J, et al. Efficacy of three different valve systems of needlefree closed connectors in avoiding access of microorganisms to endovascular catheters after incorrect handling. Crit Care Med 2008; 36: 2558–2561
- Ryder M, Fisher S, Hamilton G, Hamilton M, James G. Bacterial transfer through needlefree connectors: comparison of nine different devices. Presented at the Society for Healthcare Epidemiology of America Annual Scientific Meeting, April 2007
 Polski
Polski