dr n. med. Katarzyna Majda, lek. Sławomir Gondek
Skamex S.A.
Summary
In medical facilities, numerous medical procedures are performed daily in direct contact with patients, as well as non-medical tasks such as surface disinfection, equipment disposal, or contact with patients’ biological material. Therefore, it is recommended to use diagnostic and protective gloves, marked as both medical devices and personal protective equipment. It is crucial to select gloves with protective properties that cover the actual hazards present in the medical facility to ensure the worker’s safety.
Keywords:
medical and protective gloves ➧ European standards ➧ chemical substances ➧ permeation ➧ pictograms
In medical facilities, numerous medical procedures are performed daily in direct contact with patients, as well as non-medical procedures such as surface disinfection, equipment disposal, or contact with patients’ biological material. Therefore, it is recommended to use diagnostic and protective gloves marked as both medical devices and personal protective equipment. Manufacturers of professional medical gloves, to be able to double-mark their gloves, are required to assess their compliance with the essential requirements of the directives on medical devices and personal protective equipment. The most appropriate method to demonstrate compliance with the essential requirements is to conduct a series of tests described in the relevant European standards, namely EN 455–1, ‑2, ‑3, ‑4 (Directive 93/42/EEC for medical devices) and EN 420, EN 374–1, ‑2, ‑3, EN 388 (Directive 89/686/EEC on personal protective equipment) (see Table 1).
Medical Gloves Marking
Medical diagnostic gloves are classified as Class I medical devices (non-invasive devices). For Class I devices, the manufacturer conducts the conformity assessment themselves and may voluntarily submit to the evaluation by a notified body. In the case of personal protective equipment, most available diagnostic gloves fall under Category III (complex design, high risk) of personal protective equipment, and the conformity assessment is mandatory and conducted by a notified body, which issues the appropriate CE certificate. The European standards mentioned above for medical gloves (Table 1) [1–4] have been developed for products used in healthcare facilities during medical procedures in contact with patients.
Standards for Medical Gloves
| Poz. | Standard Number | Scope of the Standard | References |
|---|---|---|---|
| 1 | EN 455–1 | Requirements and Tests for Absence of Holes (AQL Level) | 1 |
| 2 | EN 455–2 | Requirements and Tests for Physical Properties (e.g., length, width, tensile strength) | 2 |
| 3 | EN 455–3 | Requirements and Tests for Biological Evaluation (e.g., latex protein levels, endotoxins) | 3 |
| 4 | EN 455–4 | Requirements and Tests for Determining Shelf Life | 4 |
| 5 | EN 420 | General Requirements for Protective Gloves, including Manufacturer Information on Packaging and Test Methods for Length, pH Level, etc. | 5 |
| 6 | EN 374–1 | General Requirements and Reference to Other Parts of EN 374 | 6 |
| 7 | EN 374–2 | Protection Against Microorganisms – Methodology for Determining Resistance to Penetration | 7 |
| 8 | EN 374–3 | Methodology for Determining Resistance to Chemical Permeation | 8 |
| 9 | EN 388 | Protection Against Mechanical Hazards – Determining Resistance to Puncture, Abrasion, Tear, and Cut | 0 |
Tab. 1. List of standards for gloves double-marked as medical devices and personal protective equipment [1–9].
Protective Gloves
In the case of standards for protective gloves (Table 1) [5–9], the possibility of using such medical gloves by healthcare workers was not considered during their construction. Since 2010, it has been possible to double-mark gloves, but no appropriate normative changes have been introduced. A perfect example of this is the unchanged list of 12 substances typically used in industry found in Annex A of EN 374–1 (Table 2). These substances in their pure form are not used in hospitals.
The lack of adaptation of technical standards to the requirements of the healthcare environment has led to the contentious nature of point 5.3.2 of the EN 374–1 standard, as its fulfillment is inadequate for actual use. The mentioned section includes a provision requiring gloves to achieve the second level of protection in testing using at least three substances from the list of industrial substances in Annex A (Table 2).
See rękawiczki nitrylowe sempercare
| Code Letter | Substancja chemiczna | Class |
|---|---|---|
| A | Methanol | Primary Alcohol |
| B | Acetone | Ketone |
| C | Acetonitrile | Nitrile Compound |
| D | Dichloromethane | Chlorinated Alkane |
| E | Carbon Disulfide | Organic Sulfur Compound |
| F | Toluene | Aromatic Hydrocarbon |
| G | Diethylamine | Chlorinated Alkane |
| H | Tetrahydrofuran | Heterocyclic and Ether Compound |
| I | Ethyl Acetate | Ester |
| J | n‑Heptane | Saturated Hydrocarbon |
| K | Sodium Hydroxide 40% | Inorganic Base |
| L | Sulfuric Acid 96% | Inorganic Acid |
Tab. 2The list of substances from Annex A of the EN 374–1 standard (current version) [6].
Medical gloves standardization
The European Committee for Standardization, aiming to unify the interpretation of standard provisions, through the Polish Committee for Standardization, sent a letter to the largest distributors of medical-protective gloves in Poland. According to this document, the EN 374 standard is considered fully met, both if point 5.3.2 is met and if it is excluded [10]. Notified bodies as executive bodies have adopted this principle, issuing appropriate certificates for Category III personal protective equipment in both cases; otherwise, issuing such a certificate would be impossible.
It is also worth emphasizing that meeting point 5.3.2 of the EN 374–1 standard may have little to do with actual protection against factors posing a threat to healthcare workers. The process of chemical permeation through medical gloves depends on many factors. As a result, gloves protecting against substances from Annex A may often not provide sufficient protection against substances used in medical facilities (Table 3). Depending on compliance with points 5.2.1 and/or 5.3.2 of the EN 374–1 standard, medical gloves are marked with one of two pictograms (Fig. 1A and 1B). However, the actual difference between the indicated pictograms lies in meeting or excluding point 5.3.2, and misleading information that may confuse users has appeared in the literature. In the article by A. Trzcińska, the description of the graphical symbol presented in Figure 1B (Fig. 1 in the original) states “complete protection against chemical substances” [11]. This can lead to overinterpretation and create a false sense of security when using a product that does not provide adequate protection, as there are no medical gloves that completely protect against all chemical substances, as suggested by the quoted description of the pictogram.
Due to the various types of materials from which gloves are made and the different compositions of chemical substances, there are no standard substances whose testing would guarantee protection against all possible chemical agents. Moreover, as shown above, the protection indicated by a particular pictogram may be disproportionate to the actual exposure in some professional areas, such as healthcare.
Types of medical gloves
| Types of gloves | Substances from Annex A of the EN 374–1 standard | Substances used in hospitals | ||||
|---|---|---|---|---|---|---|
| Diethylamine | Sodium hydroxide 40% | Sulfuric acid 96% | Glutaraldehyde | Mitomycin | Fluorouracil | |
| Gloves A | < 60 min | < 120 min | < 60 min | < 120 min | < 60 min | < 120 min |
| Gloves B | < 10 min | > 480 min | < 10 min | > 480 min | > 240 min | > 240 min |
| Gloves C | < 10 min | > 480 min | < 10 min | > 480 min | > 240 min | > 240 min |
Medical Protective Gloves
It should be emphasized that there is no contact with the substances listed in Annex A of EN 374–1 in medical facilities. Substances in their pure form are not present in hospitals, and moreover, routine, frequent glove changes shorten the wearing time of medical gloves to a maximum of about 30 minutes. This time is half as short as that mentioned in point 5.3.2 of EN 374–1 level 2. Additionally, the indicated protection would only be necessary in the case of full immersion in a given substance for a specified period, which further confirms that such a situation is impossible in a healthcare setting. The authors fully agree with the following opinion of Trzcińska A.: “Most manufacturers of medical-protective gloves test, in accordance with the methodology described in EN 374–3, their resistance to a range of substances not listed in Annex A of EN 374. Testing of substances outside the annex enables determination of the actual level of protection against substances characteristic for a given work area” [11]. By testing the resistance of medical-protective gloves to substances actually present in the medical environment, we are able to assess the real level of protection for healthcare workers in contact with them.
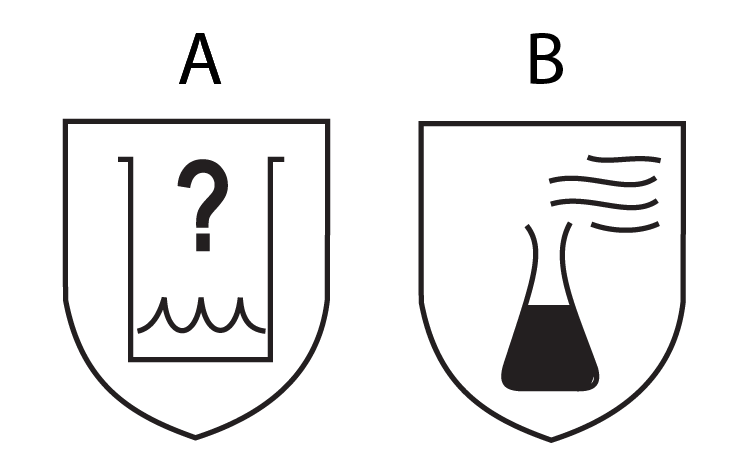
Ryc. 1. Pictograms indicating protection against chemical substances from Annex A, tested according to EN 374–3 standard: A — in accordance with point 5.2.1 of EN 374–1; B — in accordance with points 5.2.1 and 5.3.2 of EN 374–1.
Medical gloves
It is important to remember that medical protective gloves are products designed for mass use in healthcare facilities. Such gloves are primarily intended to protect personnel against microbiological hazards and chemical substances encountered on a daily basis, such as those found in disinfectants or cytostatics. In the context of existing standards, no glove used in medicine provides 100% protection against industrial substances listed in Annex A of EN 374–1, just as no glove provides mechanical protection according to EN 388 (level 0 for resistance to puncture, tear, abrasion, and cut). Therefore, it is essential to select appropriate medical gloves according to their intended use and exposure to real hazards.
Bibliography
- EN 455–1 Rękawice medyczne do jednorazowego użytku – Część 1: Wymagania i badania na nieobecność dziur.
- EN 455–2 Rękawice medyczne jednorazowego użytku – Część 2: Wymagania i badania dotyczące właściwości fizycznych.
- EN 455–3 Rękawice medyczne jednorazowego użytku – Część 3: Wymagania i badania w ocenie biologicznej.
- PN-EN 455–4 Rękawice medyczne do jednorazowego użytku – Część 4: Wymagania i badania dotyczące wyznaczania okresu trwałości.
- EN 420 Protective Gloves – Wymagania ogólne i metody badań, m.in. dostarczane przez producenta informacje, które mają zastosowanie do wszystkich rękawic ochronnych.
- EN 374–1 Rękawice chroniące przed substancjami chemicznymi i mikroorganizmami – Część 1: Terminologia i wymagania.
- EN 374–2 Rękawice chroniące przed substancjami chemicznymi i mikroorganizmami – Część 2: Wyznaczanie odporności na przesiąkanie.
- EN 374–3 Rękawice chroniące przed substancjami chemicznymi i mikroorganizmami – Część 3: Wyznaczanie odporności na przenikanie substancji chemicznych.
- EN 388 Rękawice chroniące przed zagrożeniami mechanicznymi. 10. Pismo PKN na podstawie opinii Europejskiego Komitetu Normalizacyjnego z 7.07.2016 r., adresowane do dystrybutorów rękawic medyczno–ochronnych.
- Trzcińska A.: Jakie wymagania (normy) powinny spełniać rękawiczki używane w środowisku medycznym, aby mogły zapewnić użytkownikowi ochronę przed zagrożeniami biologicznymi i chemicznymi? Zakażenia 2016, 6.
- Rękawice medyczne – wikipedia
 Polski
Polski